The Science behind Chromium Electroplating

Introduction
Chromium electroplating is a transformative process used to coat metal surfaces with a thin layer of chromium, delivering a durable, lustrous, and corrosion-resistant finish. Known for its mirror-like shine, chromium electroplating not only enhances the appearance of objects but also strengthens them against wear and rust. Here’s a deeper look into the science behind this versatile finishing technique.
1. What is Chromium Electroplating?
Chromium electroplating is a process that uses an electric current to apply a chromium layer onto a metal object, typically made from steel or other metals like nickel or copper. This layer can range in thickness depending on the application, from a thin decorative coat to a thicker, functional one. The electroplating process results in a surface that resists corrosion, withstands high temperatures, and is hard enough to resist scratching and abrasion.

Further reading: 5 Uses of Chromium | Uses of Chromium in Industry & Everyday Life
2. The Chemistry of Chromium Electroplating
The process begins by immersing the metal object into a solution of chromium ions, usually in the form of hexavalent chromium (Cr⁶⁺), a common industrial solution. When an electric current is applied, chromium ions in the solution are reduced and deposited onto the object as metallic chromium. This reduction reaction causes chromium to bind tightly to the surface, forming a thin yet extremely durable coating. The solution chemistry and careful control of temperature, current density, and plating time all influence the final properties of the chromium layer.
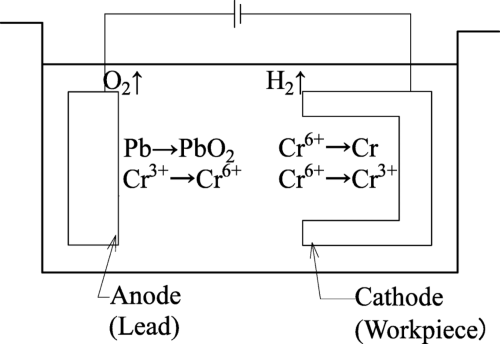 [1]
[1]
3. Types of Chromium Electroplating: Decorative vs. Hard Chromium
There are two primary types of chromium electroplating: decorative and hard chromium plating.
- Decorative Chromium Plating: Used on consumer products like faucets, car parts, and appliances, decorative chromium is typically a thin layer that gives the object a sleek, reflective finish. This type often includes an underlayer of nickel for enhanced durability and is mainly used for aesthetic purposes and light corrosion resistance.
- Hard Chromium Plating: Also known as industrial chromium plating, this type is much thicker and is used in applications requiring extreme wear resistance, such as machinery parts, hydraulic rods, and molds. Hard chromium plating significantly extends the life of components subjected to high stress, temperature, and friction.
4. Key Benefits of Chromium Electroplating
Chromium’s unique properties make it a preferred coating for various applications. Here’s why it stands out:
- Corrosion Resistance: The chromium layer creates a protective barrier against rust and oxidation, making it suitable for environments where exposure to moisture or chemicals is common.
- Hardness and Durability: Chromium plating is one of the hardest industrial coatings available, protecting parts from scratches, dents, and other wear and tear.
- Aesthetic Appeal: With its highly reflective, mirror-like finish, chromium electroplating provides a desirable appearance for consumer products, from automotive parts to household fixtures.
- Heat Resistance: Chromium plating can tolerate high temperatures, making it ideal for applications in heat-intensive environments like engines and machinery.
5. The Chromium Electroplating Process
Chromium electroplating involves several carefully controlled steps:
- Preparation and Cleaning: The substrate (usually a metal like steel or nickel) is meticulously cleaned to remove any oils, dirt, or oxidation that could affect adhesion. This may involve degreasing, rinsing, and sometimes acid cleaning.
- Bath Immersion: The cleaned substrate is immersed in a plating bath containing a solution of chromium ions, typically in the form of hexavalent chromium (Cr⁶⁺) or, increasingly, trivalent chromium (Cr³⁺) due to environmental considerations.
- Electroplating with Electric Current: When an electric current is applied, chromium ions in the solution are reduced and deposit onto the surface of the substrate, forming a thin, cohesive coating. The duration, current density, and temperature in this step determine the thickness and properties of the final layer.
- Post-Plating Finishing: Once electroplating is complete, the object is rinsed and polished to ensure a smooth, shiny finish. Additional steps like buffing or heat treatment may be applied, especially if the object requires a specific hardness or aesthetic.
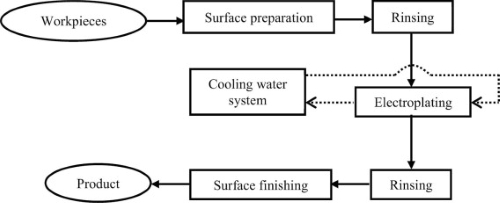 [2]
[2]
6. The Environmental and Safety Considerations
While chromium electroplating offers significant industrial advantages, the process involves certain environmental and safety risks, primarily related to the use of hexavalent chromium, a known toxic substance. Overexposure to hexavalent chromium can be hazardous to both workers and the environment. To address these concerns, industries are shifting toward trivalent chromium (Cr³⁺) plating, a safer alternative that reduces toxicity while still providing comparable durability and corrosion resistance.
7. Innovations in Chromium Electroplating Technology
Recent advances have aimed to improve both the efficiency and environmental impact of chromium electroplating. Newer methods focus on reducing energy use, minimizing hazardous waste, and increasing deposition rates for a more consistent coating. Innovations like pulse plating, which varies the electric current during plating, have helped enhance the quality of the chromium layer and reduce environmental hazards associated with traditional methods.
Conclusion
Chromium electroplating is a sophisticated process combining chemistry, engineering, and aesthetics to create products with superior durability, shine, and resistance to corrosion. Whether in decorative applications or high-performance industrial settings, chromium plating transforms the longevity and look of everyday items and critical machinery parts alike.
As new technologies emerge, the industry continues to evolve, making chromium electroplating safer and more efficient while preserving its essential benefits in both industrial and consumer applications. For more chromium products, please check Advanced Refractory Metals (ARM).
Reference:
[1] Ishizuka, Naoko & Yamada, Takayuki & Izui, Kazuhiro & Nishiwaki, Shinji. (2020). Topology optimization for unifying deposit thickness in electroplating process. Structural and Multidisciplinary Optimization. 62. 10.1007/s00158-020-02574-8.
[2] Surasit Tanthadiloke, Paisan Kittisupakorn, Iqbal M. Mujtaba, Modelling and Design a Controller for Improving the Plating Performance of a Hard Chromium Electroplating Process, Editor(s): Jiří Jaromír Klemeš, Petar Sabev Varbanov, Peng Yen Liew, Computer Aided Chemical Engineering, Elsevier, Volume 33, 2014, Pages 805-810, https://www.sciencedirect.com/science/article/pii/B9780444634566501356
{{item.content}}
LEVE A REPLY
{{item.children[0].content}}
{{item.content}}






