Refractory Metal Powders Are Expected to Become Raw Materials for 3D Printing
What is 3D printing?
3D printing is also known as additive manufacturing. Similar to the powder metallurgy processes we use today, metal devices manufactured with this technology are based on metal powders, such as ceramic powders and metal powders. The difference is that the powder is not sintered together, but formed by "printing" sections of the parts onto the powder with special adhesives, via the nozzle.
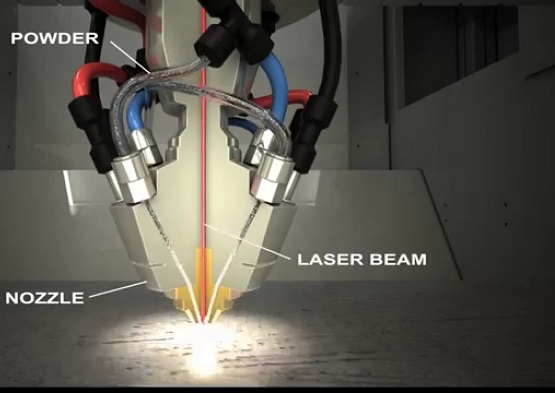
3D Printing
Refractory Metal Powders Are Expected To Become Raw Materials For 3D Printing
One of the difficulties with 3D printing is the use of refractory metals, especially those with high melting points such as tungsten, chromium, and rhenium. For years, scientists around the world have been working on new processes that can be cost-effective and meet desired performance requirements.
Scientists have developed a new technology that uses 3D printing to create complex nanoscale metal structures. The technology could be used in a variety of applications, from creating 3D logic circuits on tiny computer chips to engineering ultralight aircraft components, which can create a variety of new nanomaterials with different properties.
In 3D printing, objects are built layer by layer, allowing the creation of products that do not require conventional reduction methods such as etching or milling. Materials scientists at the California Institute of Technology (Caltech) have designed an ultra-thin three-dimensional structure in a 3D printing machine. The three-dimensional structure's beams are the only nanoscale, which is too small to be seen with the naked eye.
3D Printing of Ceramics
The new 3D group prints the structures of a variety of materials from ceramics to organic compounds. In addition, scientists are working hard to make breakthroughs in the 3D printing of refractory metals like tungsten and titanium, especially when trying to create tiny powders that are less than 50 microns in size or about half the width of a hair.
The scientists glued nickel and organic molecules together to form a liquid that looked a lot like cough syrup. They used computer software to design a structure and then built it by switching liquids using a two-photon laser. The laser creates stronger chemical bonds between organic molecules, hardening them into building blocks of the structure. And since these molecules are also bound to nickel atoms, nickel is bound to the structure. In this way, the team was able to print a three-dimensional structure that was originally a mixture of metal ions and nonmetallic organic molecules.
The structure is then placed in an oven and slowly heated to 1000 ° c in a vacuum chamber. The temperature is well below the melting point of nickel (1455 ° c), but it is hot enough to evaporate the organic material in the structure, leaving only the metal behind. The heating process known as pyrolysis also fuses metal particles.
In addition, as the process evaporates a large amount of structural material, it shrinks in size by 80% but retains its shape and proportions. The ultimate shrinkage is what makes the structure so small. In this constructed nanostructure, the printed part of the metal beam is about a thousandth the size of the tip of a sewing needle.
Scientists are still refining their techniques. Although they are just starting with nickel, they are interested in expanding into other metals commonly used in industry, such as tungsten and titanium. Scientists also hope to use the process to print other materials, including ceramics, semiconductors, piezoelectric materials, and other exotic materials.
Advanced Refractory Metals (ARM) supplies people with high-quality refractory metals products such as rhenium pellets, tantalum discs, tungsten rods, etc. all over the world. Please visit https://www.refractorymetal.org for more information.
{{item.content}}
LEVE A REPLY
{{item.children[0].content}}
{{item.content}}






