How Does Aluminum Alloy Protect Ships From Corrosion?

How Does Aluminum Alloy Protect Ships From Corrosion?
Anti-corrosion of aluminum alloy refers to the anti-corrosion of the ship hull, which is essentially related to the service life of the ship. The corrosion resistance of a ship hull depends on the corrosion resistance of raw materials and the anti-corrosion technical measures of repairing a ship hull, especially the latter.

How Does Aluminum Alloy Protect Ships from Corrosion?
Cladding Protective Layer
In the mid-1960s, the shipbuilding authorities in China tested the use of oxy-acetylene flame spray polychloro-ether plastics in aluminum alloy hulls and the use of vinyl chloride paint to prevent corrosion, they found that the former did not adhere well to the surface of the anodized aluminum plate and was easy to peel off; the latter also had poor adhesion, and the large area of paint film on the bottom of the ship was soon peeled off due to the impact of water. The results show that they are not suitable for the corrosion protection film of aluminum alloy hulls.
Later, GNA neoprene rubber was used as the coating material, the anti-corrosion property of which was very good. The combination of chloroprene rubber and aluminum alloy is very firm. It can be used continuously for more than 10 years after coating, which can significantly reduce the maintenance cost at ordinary times.
How Does Aluminum Alloy Protect Ships From Corrosion?
Paint Coating
Paint coating has been widely used in the anti-corrosion of aluminum alloy hulls and superstructures. The effect of anti-corrosion paint on aluminum alloy ship structure is closely related to the quality of raw materials and brushes.
Usually, it is necessary to do a small sample cavitation test before painting, only after qualification can be painted. The technology of epoxy zinc yellow coating is simple. However, the process of phosphating primer is complex and requires high cleaning, temperature, and humidity.
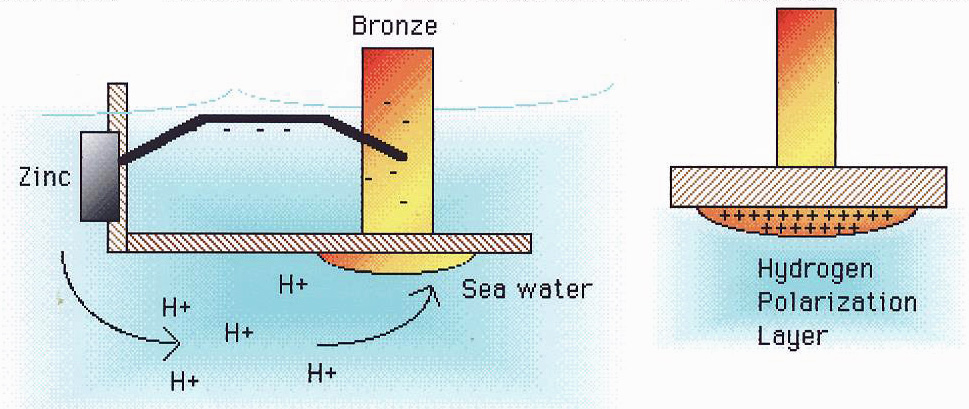
Anodic Sacrifice Protection
The vast ocean is a boundless "electrolytic tank," where seawater is a good electrolyte and aluminum ships are in it. Copper alloy thrusters, as well as steel shafts, stern shafts, and rudder blades, have higher potential. And aluminum is less positive, it's an anode. If we want to prevent the corrosion of aluminum alloy ships effectively, it is not enough to only paint the protective layer.
At present, the most effective protective measure is the comprehensive measure combining the sacrificial anode electrochemical protection and paint coating. The sacrificial anode protection is a metal and alloy below the hull waterline which is more negative than the electrode potential of the hull aluminum, and it is corroded as an anode in seawater, while the hull aluminum is protected as a cathode. The sacrificial anode protection material is usually made of low-potential zinc or zinc alloy strip and magnesium strip.
{{item.content}}
LEVE A REPLY
{{item.children[0].content}}
{{item.content}}






