Heat Resistance and Metals: A Practical Guide
Heat resistance and metals—now there’s a topic that’s kept engineers, blacksmiths, and even chefs up at night. If you’ve ever wondered why some metals can take the heat while others buckle under pressure, well, pull up a chair. With decades of experience, I’ve got a few thoughts to share.
What Makes a Metal Heat-Resistant?
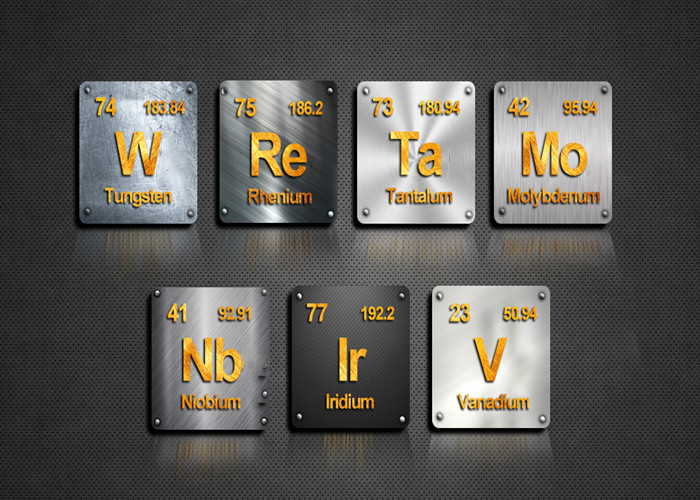
First things first: not all metals are created equal. Some, like your common aluminum foil, will melt if you stare at them too hard near a campfire. Others, like tungsten, laugh in the face of a blowtorch. So, what's the secret?
It boils down to three big factors:
- Melting Point – This is the obvious one. The higher the melting point, the more heat a metal can take before turning into a puddle. Tungsten, for example, melts at a blistering 3,422°C (6,192°F), while lead calls it quits at a measly 327°C (621°F).
- Thermal Conductivity – some metals spread the heat out rather quickly (as in copper). That can be a good or bad thing. Others, stainless steel for instance, resist thermal conduction-thus better at high temperatures
- Oxidation Resistance – ever hear of iron rusting when it gets hot? That's an oxidation reaction. Metals like chromium form a protective oxide layer over themselves that won't let the metal crumble under heat.
What Happens When Metals Get Too Hot?
Even the toughest metals have their limits. Push them too far, and you’ll see:
- Creep--The metal slowly deforms under stress, like a sagging pipe in a steam plant.
- Thermal Expansion--Metals expand when heated (ever heard a "ping" from a hot engine cooling down?). In tight machinery, that can cause big problems.
- Strength--In fires, some structural beams may collapse unexpectedly. That is because some metals lose strength when heated.
How to Improve Heat Resistance
Fortunately, we're not limited to what Mother Nature provided. Following is how we strengthen metals for high heat:
- Alloying – Combining metals (such as adding chromium to steel) increases heat resistance.
- Coatings – Refractory or ceramic coatings provide a heat shield.
- Heat Treatment – Carefully controlled heating and cooling can modify a metal's characteristics for improved performance.
Common Heat-Resistant Metals and Where You’ll Find Them
Now, let’s talk about the usual suspects in the heat-resistant lineup:
- Stainless Steel (especially grades 304 and 316) – Good up to about 870°C (1,600°F). You’ll find it in exhaust systems, industrial ovens, and even your kitchen sink.
- Titanium - Light and strong, can tolerate temperatures up to 1,650°C (3,000°F) in short periods of time. Jet engines adore it.
- Nickel Alloys (Inconel, Hastelloy) - These are the heavy guns, resistant to heat, corrosion, and pressure. Rocket nozzles and nuclear reactors take a bow.
- Tungsten - Melting point master. Filaments in light bulbs and welding electrodes.
Heat resistance isn’t just about picking the right metal—it’s about understanding how that metal behaves under stress, over time, and in real-world conditions. Whether you’re designing a furnace or just curious why your frying pan doesn’t melt, remember: metals, like people, have their limits.
Table 1: Common Examples of Heat-Resistant Metals
|
Material |
Melting Point(°C) |
Max Service Temp(°C) |
Key Properties |
Common Applications |
|
3,422 |
2,500 (inert atm) |
Highest MP, radiation shield, brittle |
Rocket nozzles, filaments, armor |
|
|
3,180 |
2,200 |
Creep-resistant, rare/expensive |
Jet turbine blades, satellite thrusters |
|
|
3,017 |
2,500 (vacuum) |
Corrosion-proof, biocompatible |
Chemical reactors, surgical implants |
|
|
Molybdenum (Mo) |
2,623 |
1,800 (oxidizing) |
Thermal conductivity, strong |
Furnace electrodes, aerospace parts |
|
Niobium (Nb) |
2,468 |
1,400 |
Superconductive, lightweight |
Particle accelerators, jet engine linings |
|
Hafnium (Hf) |
2,233 |
1,700 |
Neutron absorber, ductile |
Nuclear control rods, plasma cutters |
Note: Refractory metals feature ultra-high melting points (>2000°C) but often require protective coatings for oxidation resistance, making them ideal for extreme environments like space and nuclear applications.
Further reading: A List of Heat-Resistant Materials
Table 2: Common Examples of Heat-Resistant Alloys
|
Material |
Melting Point (°C) |
Max Service Temp (°C) |
Key Properties |
Common Applications |
|
Nickel Alloys |
1,000 |
Creep-resistant, oxidation-resistant |
Jet engines, gas turbines, nuclear |
|
|
Hastelloy X |
1,200 |
Excellent hot corrosion resistance |
Combustors, industrial furnaces |
|
|
Stainless Steel |
304 Stainless |
870 |
Good oxidation resistance, weldable |
Exhaust systems, food processing |
|
316 Stainless |
900 |
Superior corrosion resistance |
Chemical tanks, marine environments |
|
|
Titanium Alloys |
Ti-6Al-4V |
600 |
High strength-to-weight ratio |
Aerospace structures, medical implants |
|
Ti-6242 |
800 |
Improved creep resistance |
Jet engine components |
|
|
Cobalt Alloys |
Haynes 188 |
1,100 |
Oxidation-resistant, fatigue-resistant |
Turbine blades, afterburners |
|
Nickel Alloys |
Inconel 718 |
1,000 |
Creep-resistant, oxidation-resistant |
Jet engines, gas turbines, nuclear |
|
Hastelloy X |
1,200 |
Excellent hot corrosion resistance |
Combustors, industrial furnaces |
Note: High-temperature alloys balance strength, oxidation resistance, and cost, serving widely in aerospace and industrial systems up to ~1300°C.
Further reading: High-Temperature Alloys: Inconel, Hastelloy, and Beyond
FAQs (Frequently Asked Questions)
1. Why does aluminum foil melt so easily compared to steel?
Aluminum has a much, much lower melting point (~660°C) compared to steel (~1,370°C–1,530°C). Good for baking potatoes but bad at holding molten metal.
2. Is there any metal that can withstand lava temperatures?
Most lava ranges between 700°C–1,200°C, so nickel alloys (such as Inconel) or tungsten could stand it—if only briefly, and would still struggle if exposed for any longer than that.
3. Why do some metals have this red hot, glowing phase before they melt?
That's incandescence—heat makes them emit light. The color (red, orange, white) tells you how hot they are, like a built-in thermometer.
{{item.content}}
LEVE A REPLY
{{item.children[0].content}}
{{item.content}}






