11 Interesting Facts about Gallium

Introduction
Gallium the element comes with a combination of properties that make it both scientifically intriguing and industrially indispensable. Discovered in 1875 by French chemist Paul-Émile Lecoq de Boisbaudran, Ga quickly gained attention for its ability to melt in your hand. Beyond its novelty, gallium has become a vital component in cutting-edge technologies, from semiconductors and LEDs to solar cells and medical imaging. Let’s explore more fascinating characteristics, features, and uses of this unique element.
 [1]
[1]
Discovery and History
1. Gallium Melts in Your Hand
One of Ga’s most distinctive and visually captivating properties is its low melting point, which sits at just 29.76°C (85.57°F). This means gallium is solid at room temperature but will melt easily if held in the hand, as body temperature is high enough to turn it into a liquid. This property makes Ga a popular curiosity among science enthusiasts and educators, who use it to demonstrate phase changes in a way that’s accessible and fascinating.
2. Gallium Expands When It Freezes
Another intriguing aspect is its behavior when transitioning from a liquid to a solid state. Most materials contract when they freeze, but gallium does the opposite—it expands by about 3.1% upon solidification. This expansion is similar to the behavior of water, which also expands as it freezes. Because of this, Ga cannot be stored in glass containers when solid, as the expansion could cause the container to crack.
3. Gallium Was Predicted Before Its Discovery
Before Ga was discovered, its existence was predicted by the Russian chemist Dmitri Mendeleev, who is famous for developing the periodic table. Mendeleev noted a gap in his table and hypothesized the existence of an element with properties similar to aluminum. He referred to this element as "eka-aluminum," and when gallium was discovered, it fit his predictions almost perfectly. This discovery validated Mendeleev’s periodic law and helped to further establish the periodic table as a powerful predictive tool in chemistry. Related video: https://www.youtube.com/watch?v=tjPCrh7cdAo
Unique Properties of Gallium
4. Low Melting Point and High Boiling Point
The melting point is one of its most striking properties, but what makes it even more remarkable is its extremely high boiling point of 2400°C (4352°F). This combination of a low melting point and high boiling point gives Ga an unusual stability across a wide temperature range, making it useful in various high-temperature applications. Its low melting point allows it to be used in heat-sensitive processes, while its resistance to vaporization at high temperatures makes it suitable for more extreme environments.
5. Semiconductor Properties
Gallium is a key player in the semiconductor industry, where it forms compounds like gallium arsenide (GaAs) and gallium nitride (GaN). These materials have exceptional electronic properties that make them ideal for high-speed devices. Compared to silicon, which is the most commonly used semiconductor material, gallium-based semiconductors are more efficient in high-frequency applications, such as radar systems, satellite communications, and mobile phones.
6. Resistant to Corrosion
Another feature is its resistance to corrosion in air and water. When exposed to oxygen, gallium forms a thin oxide layer that protects it from further oxidation and degradation. This corrosion resistance makes Ga a durable choice in environments where long-term stability is required, such as in electronics and certain industrial applications.
Common Uses and Applications
7. Semiconductors
One of the most significant uses is in the production of semiconductors. Gallium arsenide (GaAs) and gallium nitride (GaN) are widely used in high-speed electronics, including transistors, integrated circuits, and power amplifiers. GaAs is preferred over silicon in applications where speed and efficiency are paramount, such as in satellite communication, radar systems, and high-frequency microwave devices. GaN, on the other hand, is widely used in the production of power transistors and is considered a key material for the future of high-efficiency electronics.
Related reading: Gallium Arsenide Wafer VS. Silicon Wafer
8. Light Emitting Diodes (LEDs)
Gallium nitride (GaN) is also essential in the creation of LEDs. LEDs made from GaN are known for their brightness and energy efficiency, making them the preferred choice for everything from display screens to general lighting systems. GaN allows for the production of blue and green LEDs, which, when combined with phosphors, can produce white light. Gallium phosphide (GaP) is used to produce red, green, and yellow LEDs, which are commonly used in traffic signals, indicator lights, and display panels.
9. Solar Cells
Gallium plays a crucial role in the development of high-efficiency solar cells, especially those used in space applications. Gallium arsenide (GaAs) solar cells are favored for satellites and spacecraft because of their superior efficiency and resistance to radiation, which makes them more durable and reliable in the harsh conditions of space. GaAs solar cells are also used in concentrated photovoltaic systems, where they can achieve higher efficiencies than traditional silicon cells.
10. Medical Imaging
Radioactive isotopes of gallium, such as gallium-67 and gallium-68, are used in medical imaging techniques. Gallium-67 is used in nuclear medicine to detect infections, inflammation, and certain types of cancer, as it accumulates in areas with high metabolic activity. Gallium-68 is used in positron emission tomography (PET) scans, providing detailed diagnostic images, particularly for detecting tumors and assessing organ function.
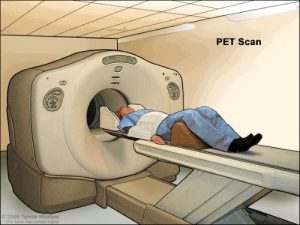
11. Thermometers and Barometers
Gallium is also used as a non-toxic alternative to mercury in thermometers and barometers. Its low melting point allows it to accurately measure temperature changes, while its non-toxic nature makes it a safer option in applications where mercury would pose environmental or health risks. Gallium-based thermometers are commonly used in laboratories and industrial settings where precision is key.
Conclusion
Gallium possesses unique properties and a wide range of uses. From its role in semiconductors and LEDs to its applications in solar cells and medical imaging, Ga’s low melting point, high boiling point, and corrosion resistance set it apart. Whether you’re interested in its scientific novelty or its industrial importance, Ga continues to shape the future of high-tech applications. For more details, please check Advanced Refractory Metals (ARM).
Reference:
[1] Gallium. (2024, August 31). In Wikipedia. https://en.wikipedia.org/wiki/Gallium
[2] National Cancer Institute (2024, September 10). PET scan. Retrieved September 10, 2024, from https://www.cancer.gov/publications/dictionaries/cancer-terms/def/pet-scan
{{item.content}}
LEVE A REPLY
{{item.children[0].content}}
{{item.content}}






